Free Energy Formula
The change in free energy ΔG is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. For a reversible process that does not involve external work we can express the change in free energy in terms of volume pressure entropy and temperature thereby eliminating ΔH from the equation for ΔG.

Entropy Free Energy And Equilibrium Chapter Ppt Download
It is expressed in two forms.

Free energy formula. The following equation relates the standard-state free energy of reaction with the free energy at any point in a given reaction not necessarily at standard-state conditions. It is defined by the Gibbs equation. DG DH - TDS For a spontaneous process at constant temperature and pressure DG must be negative.
Gibbs Energy in Equilibria. If the formula for calculating the energy stored in a capacitor E 12CV2 is applied to the earth it turns out that the ambient medium contains 16 x 1011 joules or 45 megawatt-hours of electrical energy. Ergs T is the absolute temperature kelvins of the surroundings modelled as a heat bath S is the entropy of the system SI.
The maximum work done is the amount of energy produced given by the decrease. The relationship between the free energy of reaction at any moment in time G and the standard-state free energy of reaction G o is described by the following equation. You must convert your standard free energy value into joules by multiplying the kJ value by 1000.
F is the Helmholtz free energy sometimes also called A SI. Click to see full answer. G G o RT ln Q In this equation R is the ideal gas constant in units of Jmol-K T is the temperature in kelvin ln represents a logarithm to the base e and Q is the reaction quotient at that moment in time.
Free energy is used to determine how systems change and how much work they can produce. The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings. Free Energy ΔG is equal to the maximum amount of work a system can perform on its surroundings while undergoing a spontaneous change.
The Helmholtz free energy F sometimes called the work function and the Gibbs free energy G. ΔG ΔGo RTlnQ ΔG free energy at any moment ΔGo standard-state free energy. The enthalpy and entropy changes of a reaction together with the temperature at which the reaction takes place.
Free energy has the dimensions of energy and its value is determined by the state of the system and not by its history. Gibbs free energy G is defined as G H - TS where H T and S are the enthalpy temperature and entropy. Lets take a step back and look at each component of this equation.
Were going to explore Gibbs free energy a little bit in this video in particular its usefulness in determining whether a reaction is going to be spontaneous or not which is super useful in chemistry and biology and it was defined by Josiah Willard Gibbs and what we see here we see this famous formula which is going to help us predict spontaneity and it says that the change in Gibbs free energy. Also by Gibbs formula we determine whether a reaction is favored or disfavored. Furthermore the Gibbs energy is the thermodynamic potential that a system minimizes when a system reaches chemical equilibrium at a constant temperature and pressure.
Gibbs Free Energy Formula Gibbs Free Energy Formula Willard Gibbs defined a function known as Gibbs energy G to calculate the changes in entropy and enthalpy values. With a potential of 360000 volts the earth constitutes a capacitor of 25 farads farads coulombsvolts. How to make free energy with electric motor 100 new formula 10 hp single and three phase motor New Technology.
Ergs U is the internal energy of the system SI. Gibbs Free Energy Formula. Joules per kelvin CGS.
In a practical and frequently used form of Gibbs free energy change equation Δ G is calculated from a set values that can be measured by scientists. Delta G Delta H T Delta S. The formula of Gibbs free energy is.
Gibbs free energy denoted G combines enthalpy and entropy into a single value. The SI unit for Gibbs energy is the kilojoule.
Gibbs Free Energy Graph
The standard Gibbs free energy of formation Gf of a compound is the change of Gibbs free energy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states the most stable form of the element at 1 bar of pressure and the specified temperature usually. ΔH -8904 kJ mol-1.
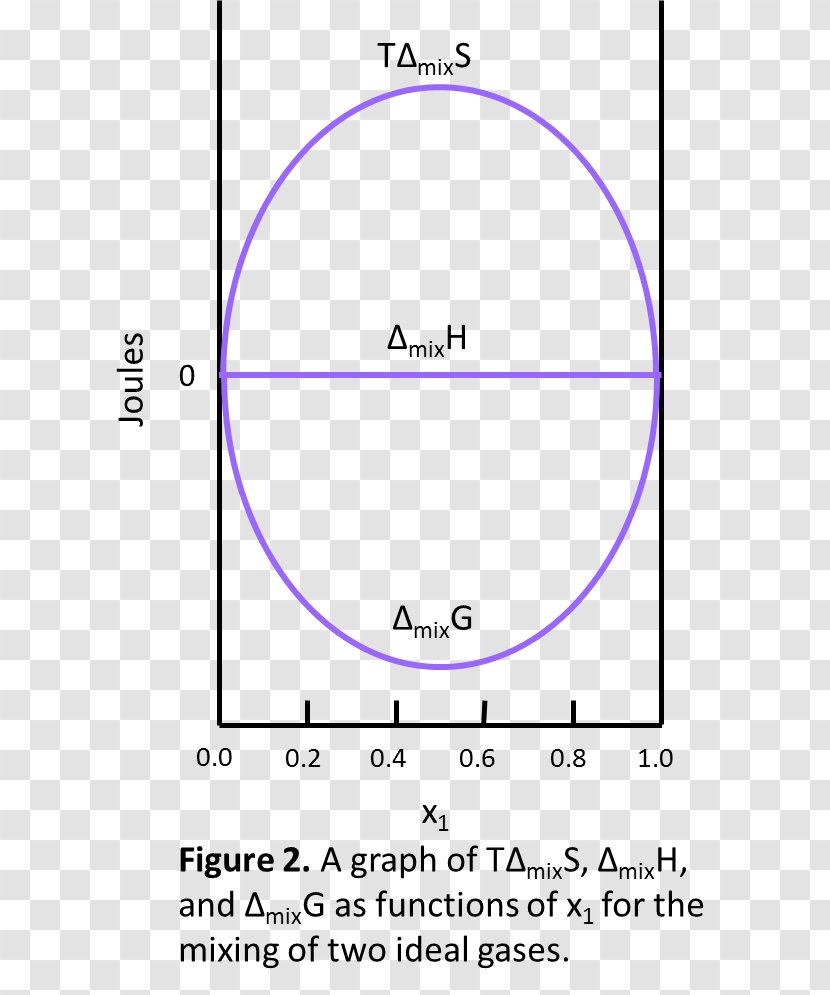
Gibbs Free Energy Entropy Of Mixing Mixture Enthalpy Ideal Solution Chemistry Transparent Png
ΔG ΔH - TΔS.
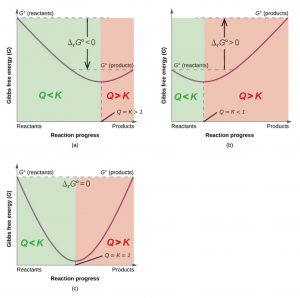
Gibbs free energy graph. Free Energy and Free Energy Change the Gibbs free energy G is used to describe the spontaneity of a process. Substitute the values of K and Q into Equation 4 to obtain ΔG for the reaction under nonstandard conditions. D G D H - T D S.
Google Classroom Facebook Twitter. ΔG can predict the direction of the chemical reaction under two conditions. Δ G Δ H T Δ S.
If you think about it a negative standard Gibbs energy of formation of which this is an example can in fact be considered a definition of molecular stability. C H The gradient of this graph is equal to-S. G G o RT ln Q In this equation R is the ideal gas constant in units of Jmol-K T is the temperature in kelvin ln represents a logarithm to the base e and Q is the reaction quotient at that moment in time.
Willard Gibbs 1873 available energy free energy graph which shows a plane perpendicular to the axis of v and passing through point A which represents the initial state of the bodyMN is the section of the surface of dissipated energyQε and Qη are sections of the planes η 0 and ε 0 and therefore parallel to the axes of ε internal energy and η respectively. The Gibbs free energy change ΔG and how its related to reaction spontaneity and equilibrium. The free energy change D G is equal to -T D S univ and it applies just to a system itself without regard for the surroundings.
Gibbs free energy and spontaneous reactions. 2H 2g 1 2O 2g H 2Ol with ΔGo 2372kJ. A Using the equilibrium constant expression for the given reaction we can calculate Q.
So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in. Standard vs Non-Standard Gibbs Free Energy - Graphs. Energy that is not extracted as work is exchanged with the surroundings as heat.
ΔG -8904 - 298-02442 -8176 kJ mol-1. The relationship between the free energy of reaction at any moment in time G and the standard-state free energy of reaction G o is described by the following equation. Finally they will also be met when we create a graph of the standard Gibbs free energy change versus temperature and utilize the slope and y-intercept to find the standard entropy and enthalpy change of the reaction.
The work is done at the expense of the systems internal energy. Gibbs free energy denoted G combines enthalpy and entropy into a single value. Δ G Δ H T Δ S.
The behavior of GN PT particularly as a function of P and T can signify a phase transition and can tell us some of the thermodynamic properties of different phases. It is easy as long as you remember to convert the entropy change value into kJ. Some of the ice polymorphs.
The reversible condition implies wmax and qmin. Please help improve this article by introducing citations to additional sources. The Gibbs free energy is a particularly important function in the study of phases and phase transitions.
Advanced Level Chemistry TutorialsGibbs free energy graphs and equilibrium sසමතලතතව සහ ගබස ශකත ප. This graph shows how the free-energy change for formation of ammonia varies with temperature above 240 K. ½ N2g 3 2 H2g NH3g Applying the equation of a straight line y mxc to the G H - TS equation.
Then using the rate constant and temperature values the standard Gibbs free energy change will be calculated. In this screencast John Holman explains the variation of Gibbs Energy change with temperature. We can predict whether a reaction will occur spontaneously by combining the entropy enthalpy and temperature of a system in a new state function called Gibbs free energy G.
Q NH 3 2 N 2 H 2 3 0021 2 200 700 3 64 10 7. G H - T D S. It is defined by the Gibbs equation.
The change in free energy ΔG is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. Standard vs Non-Standard Gibbs Free Energy - Graphs. Note that this refers to liquid water the standard state of H 2 O at 25.
The Gibbs free energy is the maximum amount of non-expansion work that can be extracted from a closed system. Endergonic exergonic exothermic and endothermic.
